Ph From Ka and Molarity Calculator
Solving for K a. We all enjoy a cool dip in a swimming pool on a hot day but we may not realize the work needed to keep that water safe and healthy.

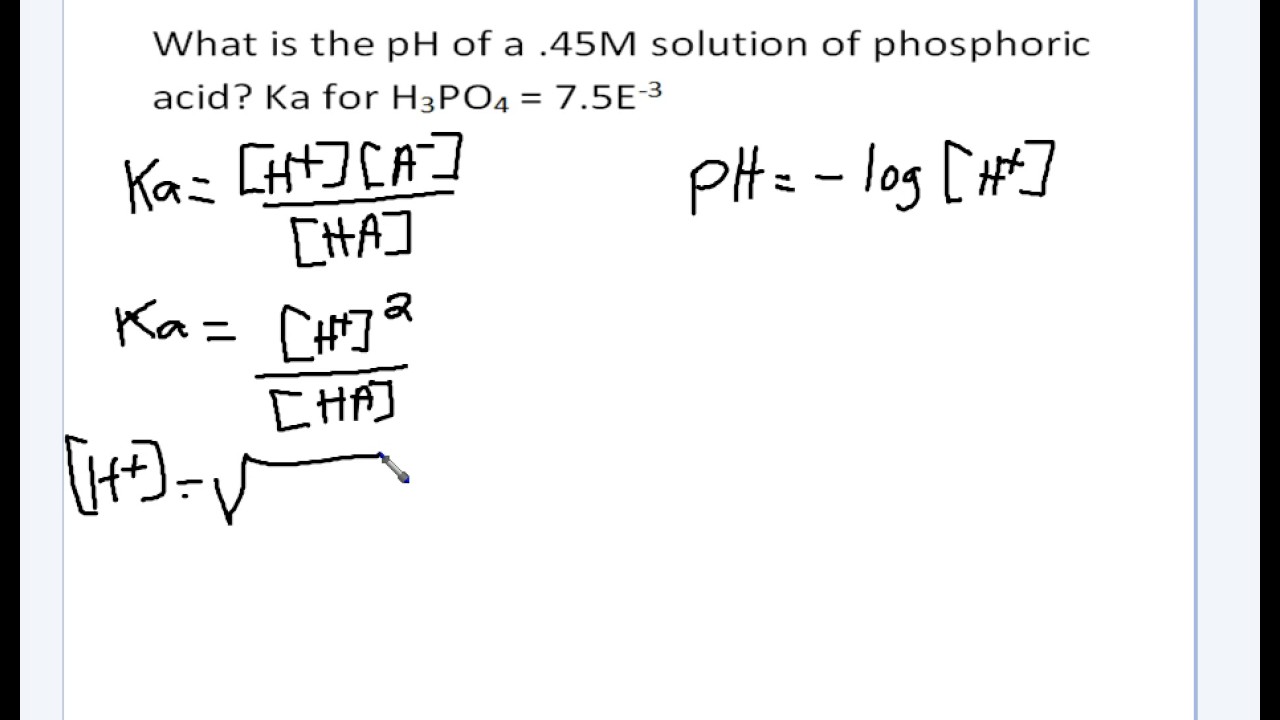
Using Ka To Calculate Ph Youtube
Thus the pH of the acid is 27.
. Online pH Calculator Weak acid solution. To calculate pH all you need is the H ion concentration and a basic calculator because it is a very straightforward calculation. Construct an ICE table using the following reaction equation.
What happens is that some teachers will. For a pu02M ceHNO2 solution puK_a 45 times 10-4 calculate the concentration of the ceH and ceNO2- ions ceHNO2 molecules and the cepH of the solution. Have another read of our.
Example Problem 2 - Calculate the Ka of a Weak Acid from pH Calculate the Ka value of a 0021 M aqueous solution of nitrous acid HNO2 with a pH of 328. H Aaq H 2Ol H 3O aq A aq By definition the acid dissociation constant Ka will be equal to. Use the concentration of H 3 O to solve for the concentrations of the other products and.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Answer 1 of 2. Formula to calculate pH from molarity.
Build your own widget. To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter molarity. The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution.
We all enjoy a fresh dip in a pool on a hot day but we can not do the work necessary to keep the water safe and healthy. Calculate the K a of a weak acid given the pH and molarity Problem 1. Calculate ph from ka and molarity Learning Objectives Perform calculations to determine pH of salt solutions if or are known.
To find pH for a given molarity you need to know how to work with logarithmic equations and a pH formula. Notice that a generic weak acid is used symbolized by the formula HA. H A x 56.
Is impossible to answer to your question without knowing is you are considering an acid a base or a salt. If you need to determine the pH of a solution from the pKa of the acid dissolved which can be determined in turn from its acid dissociation constant Ka you can use the Henderson-Hasselbach equation. You just need to know the equilibrium concentration of the acid and its conjugate base.
The pH Equation. The ideal pH for a pool is. A good reference that can walk you through the calculations for pH and related topics can be found here at Purdue Universitys website.
Enter the chemical solution name and its concentration value in the respective input field. Create an ICE table. If anyone could give any hints or answers that would be great.
This is derived from the molarity of protons hydrogen ions or H in the solution. Find the pH of a 00020 M HCl solution. Solve for the concentration of H 3 O using the equation for pH.
The key is knowing the concentration of H ions and that is easier with strong acids than it is with weak acids. Now click the button Calculate to get the pH value. The procedure to use the pH calculator is as follows.
Finally the pH value will be displayed in the new window. Since we know that HCl is a strong acid and is 100 ionized in water therefore. Ph from ka and molarity calculator Perform calculations to determine the pH of salt solutions if or are known.
The H ion concentration must be in mol dm-3 moles per dm 3. When given the pH value of a solution solving for K a requires the following steps. Determine the K a.
The general dissociation equation for a weak acid looks like this. Set up an ICE table for the chemical reaction. Calculate the pH by taking the -log of the concentration of the H3O.
Strong acid Weak acid Strong base Weak base Acid-base mixtures. H 2O H A A H 3O. Calculate the pH value from the Ka by using the Ka to find the concentrations or molarity of the products and reactants when an acid or base is in an aqueous solution.
Calculate the pH the pOH and the molarity of all species in solution for a 245 M butanoic acid solution. No one cares what the specific acid is because the technique to be explained works for all weak acids. So the pH is calculated for the example like this.
Just as example if the molarity of HCl is. In this case the calculation is easy because the molarity for H ions is the same as the molarity of the acid. To find pH of a weak acid monoprotic solution insert concentration M and insert Ka value of the weak acid0001 is input as 1E-3 calculate.
In case of a strong acids as HCl you can use the molarity and rapidly calculate the pH. Science Chemistry QA Library Calculate the pH the pOH and the molarity of all species in solution for a 245 M butanoic acid solution. Ka H 3O A H A If you have a 11 mole ratio between the acid and the hydronium ions and between the hydronium ions and the conjugate base A then.
A 0120 M solution of a generic weak acid HA has a pH of 326. Make an ICE table recording the initial concentration change in concentration and. PH See the equations used to make this calculation.
5 H 3 O 10 p H. PH -log H. Use the pH to calculate H 3O at equilibrium which is also the change in concentration for the table.
The ideal pH for a swimming pool is around 72.


0 Response to "Ph From Ka and Molarity Calculator"
Post a Comment